Simplify Cervical Disc
The most clinically effective cervical total disc replacement (cTDR)1
The cervical disc replacement with the highest overall clinical success rate at one and two levels.1
The cervical disc replacement with the highest overall clinical success rate at one and two levels.1
Simplify Cervical Disc is the only cTDR to achieve clinical superiority at primary endpoints for 1- and 2-levels compared to anterior cervical discectomy and fusion (ACDF). 2
Simplify Cervical Disc is designed to fit patient anatomy, protect facets, and prevent overstuffing. It is designed for enhanced visualization through magnetic resonsance imaging (MRI).3
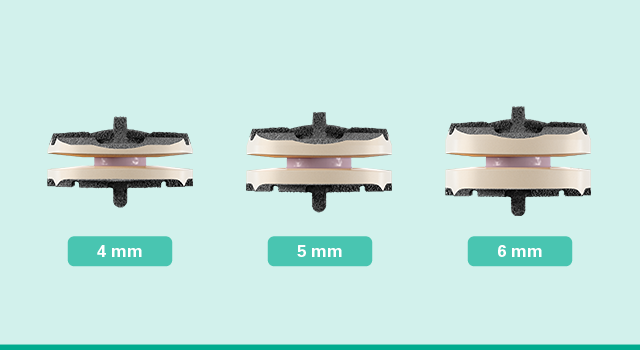 Anatomic disc heights
Anatomic disc heights
Simplify Cervical Disc features the lowest available disc heights, starting at 4mm, to better match patient anatomies and protect facet joints.
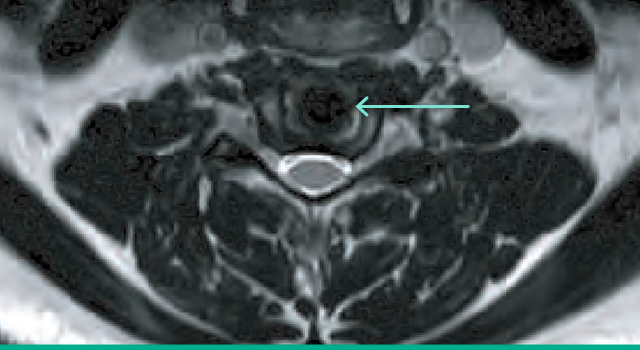 Radiologic design
Radiologic design
Simplify Cervical Disc’s PEEK endplates and ceramic core permit detailed anatomic visualization on MR imaging3 postoperatively.
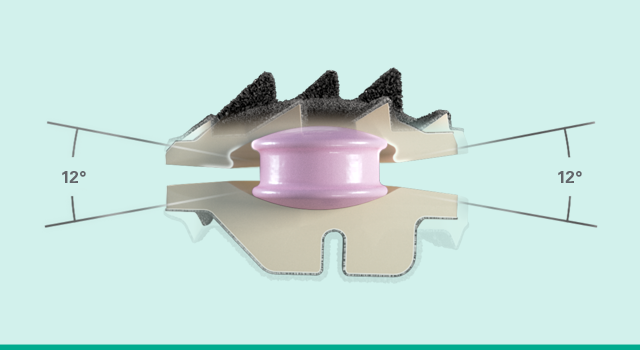 Physiologic motion
Physiologic motion
Simplify Cervical Disc’s unique biconvex, dual articulation allows for pure translation, coupled motion patterns and a variable center of rotation.
Simplify Cervical Disc has the highest overall clinical success rate for both 1 and 2-levels compared to any approved cervical disc.1
Simplify Disc was approved for 1-level use in September 2020 and achieved superiority to fusion with an overall success rate of 93.0% versus 73.6%. The 2-level approval was received in April 2021 and the Simplify Disc was again superior to ACDF- 86.7% versus 77.1%.2
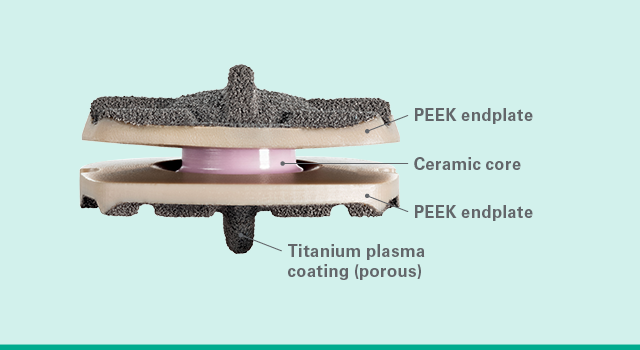 Biologic materials
Biologic materials
Simplify Cervical Disc offers nickel-free materials and metal-free articulating surfaces, significantly minimizing the risk of metal-wear debris.
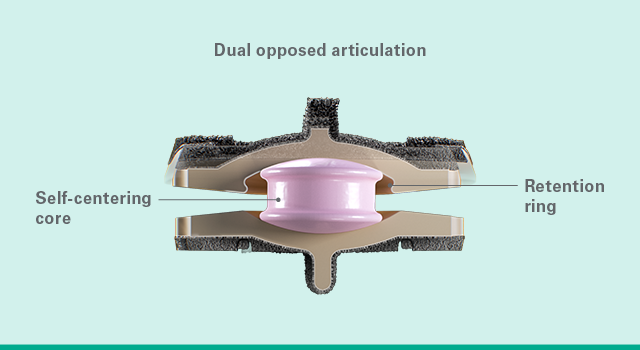 Game-changing disc
Game-changing disc
Simplify Cervical Disc, offered in 12 size combinations, is designed to prevent core expulsion and achieve vertebral fixation.
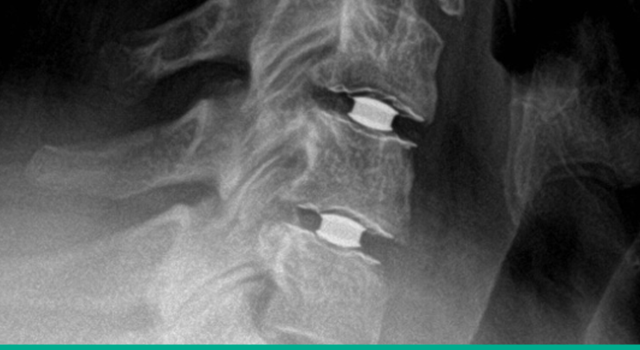 Superior clinical results
Superior clinical results
Simplify Cervical Disc achieved superiority to fusion with an overall success rate of 93.0% and a 15-point NDI improvement. 2
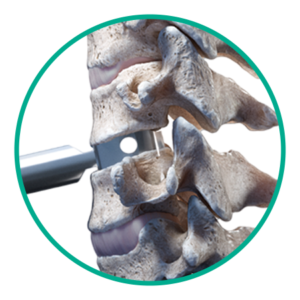
Step 1: Disc sizing
Determine the footprint and height of the disc space.
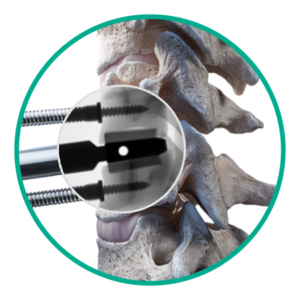
Step 2: Slot cutting
Create slot cuts for disc placement.
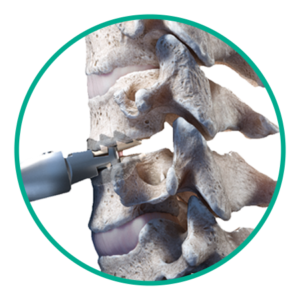
Step 3: Disc placement
Insert Simplify Cervical Disc and finalize placement.
![]()
Literature
Click here to download Simplify Cervical Disc Instructions for Use (IFU) Surgical Technique Guide, Patient Labeling, and more.
![]()
Reimbursement and coverage
Click here for reimbursement support. cTDR is considered to be a covered procedure by nearly all commercial health plans.
Watch the NuVasive Innovation Event to see our latest innovations.
Simplify Cervical Disc is not yet available in all countries.
1. Data on file. Based on review of publicly available materials at the time of this release.
2. Simplify Cervical Disc- P200022 and P200022/S003A
3. MR Conditional per ASTM F2503
Please refer to the Simplify Disc IFU and Patient Labeling for important product information, including but not limited to, Indications, Contraindications, Warnings, Precautions, Risks and Potential Adverse Effects. The IFU and Patient Labeling can be found at www.nuvasive.com/eifu.